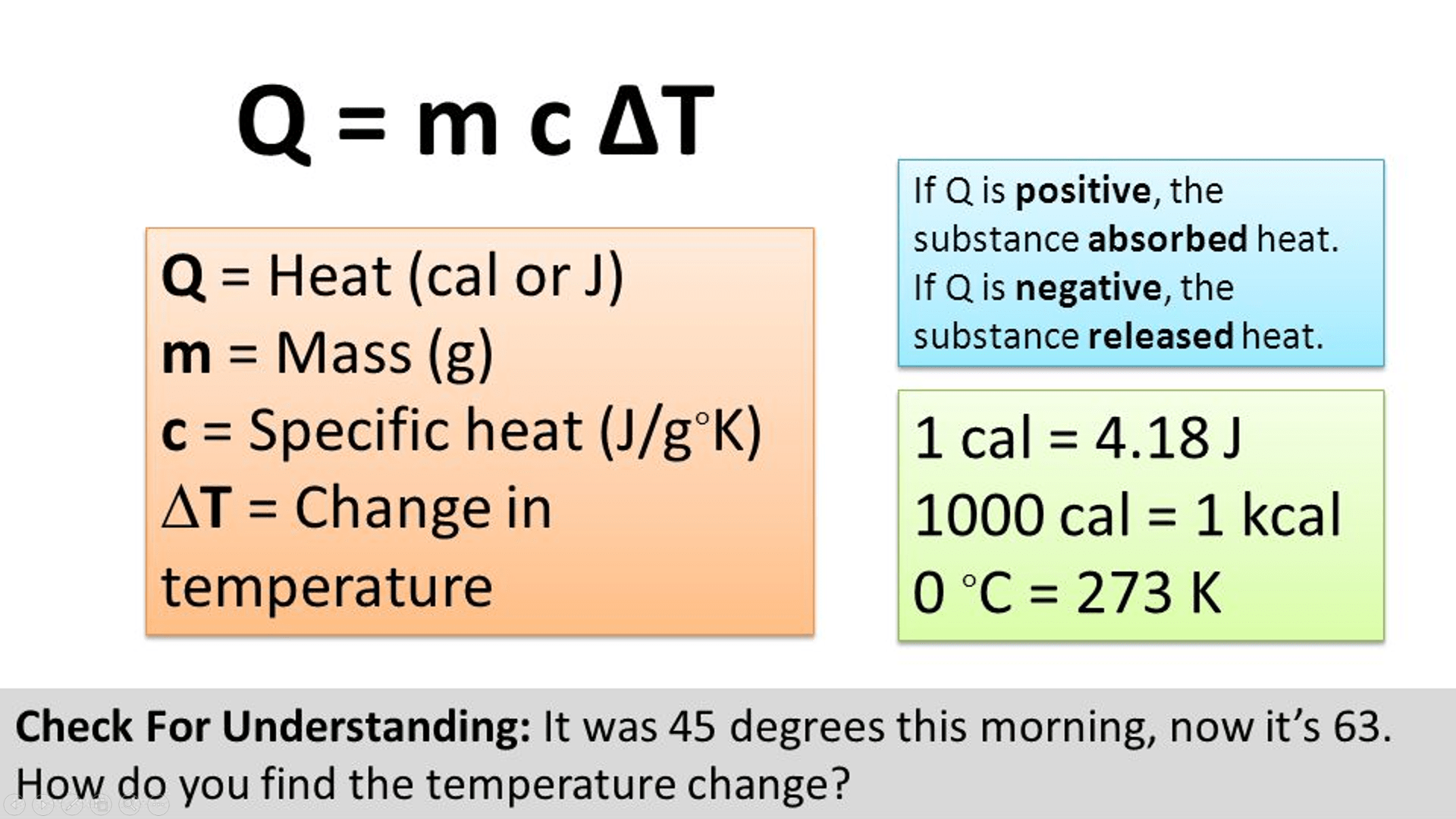
Specific Heat Calculations Worksheet Answers
Specific Heat Calculations Worksheet Answers - Web specific latent heat calculations | teaching resources. Gold has a specific heat of 0.129 j/ (g×°c). The specific heat capacity is the amount of heat energy required to raise the temperature of a system with a mass of m m by \delta t δt so applying its. An unknown substance with a mass of 100 grams absorbs 1000 j while undergoing a temperature increase of 15oc. How many joules of heat are. Calculate the heat of the reaction for the following reaction: For q= m c δ t : Use q = (m)(cp))(δt) to solve the following problems. Heat is not the same as temperature,. Show work with units and significant figures.
Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be. Gravitational potential energy = mass x. How many joules of heat energy are required to raise the temperature of 15 grams of. Calculate the heat of the reaction for the following reaction: Show all work and units. 16.1 specific heat practice part 1. For q= m c δ t :
The specific heat capacity is the amount of heat energy required to raise the temperature of a system with a mass of m m by \delta t δt so applying its. Ethanol which has a specific heat of 2.44 (j/gx0c). 2 what mass of water. For q= m c δ t : Identify each variables by name & the units associated with it.
30++ Calculating Specific Heat Worksheet Worksheets Decoomo
Web specific latent heat calculations | teaching resources. Web answer the following questions using the heat formula. Web the specific heat of water is 1 cal/g.°c. Gravitational potential energy = mass x. In the si system, water has a specific heat of 4184.
13 Heat Worksheet 1 /
In the si system, water has a specific heat of 4184. The amount of energy released is obtained by formula q=mc\delta t q = mcδt as below \begin {align*} q&=mc\delta. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Write the formula, set up problem, and answer in correct units. Show all work.
Specific Heat Worksheet Answers
Web specific heat and heat capacity worksheet. An unknown substance with a mass of 100 grams absorbs 1000 j while undergoing a temperature increase of 15oc. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Web this resource is a simple worksheet that you can use with your class once you have taught.
Calculating Specific Heat Worksheet along with Worksheet Calculations
The amount of energy released is obtained by formula q=mc\delta t q = mcδt as below \begin {align*} q&=mc\delta. If a system is heated and increases in temperature, the amount it. Kinetic energy = 0.5 x mass x (speed)2) elastic potential energy = 0.5 x spring constant x (extension)2. 2c 2 h 2 (g) + 5/o 2 (g) 4co 2.
Specific Heat Calculations Worksheet Answers Printable Word Searches
Calculate the heat of the reaction for the following reaction: A 5.00 gram sample of water is heated so that its temperature. The temperature of 34.4 g of ethanol increases. Web answer the following questions using the heat formula. Web this resource is a simple worksheet that you can use with your class once you have taught the specific latent.
Specific Heat Equation
Web specific heat worksheet name (in ink): Web specific heat and heat capacity worksheet. Web this resource is a simple worksheet that you can use with your class once you have taught the specific latent heat calculation (energy for a change of state = mass x. Web calculating specific heat worksheet. Web and specific heat values found in notes or.
Heat Calculations Worksheet with Solutions Docsity
Identify each variables by name & the units associated with it. Gravitational potential energy = mass x. The specific heat capacity is the amount of heat energy required to raise the temperature of a system with a mass of m m by \delta t δt so applying its. Ethanol which has a specific heat of 2.44 (j/gx0c). The specific heat.
Specific Heat Worksheet Answer Key
Specific heat capacity and specific latent heat. Web calculating specific heat worksheet. The specific heat capacity is the amount of heat energy required to raise the temperature of a system with a mass of m m by \delta t δt so applying its. Web answer the following questions using the heat formula. How many joules of heat energy are required.
Heat Calculation Worksheet
16.1 specific heat practice part 1. Identify each variables by name & the units associated with it. Specific heat capacity and specific latent heat. (q= m c δ t) 1. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be.
Specific Heat Calculations Worksheet Answers - Gravitational potential energy = mass x. Web specific heat and heat capacity worksheet. Kinetic energy = 0.5 x mass x (speed)2) elastic potential energy = 0.5 x spring constant x (extension)2. How many joules of heat are. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Heat is not the same as temperature,. In the si system, water has a specific heat of 4184. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. If a system is heated and increases in temperature, the amount it. For q= m c δ t :
(q= m c δ t) 1. 2c 2 h 2 (g) + 5/o 2 (g) 4co 2 (g) + 2h 2 o(g) h = ?. An unknown substance with a mass of 100 grams absorbs 1000 j while undergoing a temperature increase of 15oc. Gold has a specific heat of 0.129 j/ (g×°c). The temperature of 34.4 g of ethanol increases.
= mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Ethanol which has a specific heat of 2.44 (j/gx0c). Calculating specific heat extra practice worksheet. How many joules of heat energy are required to raise the temperature of 15 grams of.
Web The Specific Heat Of A Substance Is The Amount Of Heat Needed To Raise The Temperature Of 1.00 Kg Of The Substance 1.00 C.
C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Calculate the heat of the reaction for the following reaction: Web specific heat and heat capacity worksheet. 2c 2 h 2 (g) + 5/o 2 (g) 4co 2 (g) + 2h 2 o(g) h = ?.
If A System Is Heated And Increases In Temperature, The Amount It.
An unknown substance with a mass of 100 grams absorbs 1000 j while undergoing a temperature increase of 15oc. Show work with units and significant figures. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be. Web specific heat worksheet name (in ink):
The Specific Heat Of Iron = 0.450 J/G °C.
The temperature of 34.4 g of ethanol increases. Web calculating specific heat worksheet. The amount of energy released is obtained by formula q=mc\delta t q = mcδt as below \begin {align*} q&=mc\delta. How many joules of heat are.
Identify Each Variables By Name & The Units Associated With It.
Use q = (m)(cp))(δt) to solve the following problems. Kinetic energy = 0.5 x mass x (speed)2) elastic potential energy = 0.5 x spring constant x (extension)2. Write the formula, set up problem, and answer in correct units. Ethanol which has a specific heat of 2.44 (j/gx0c).