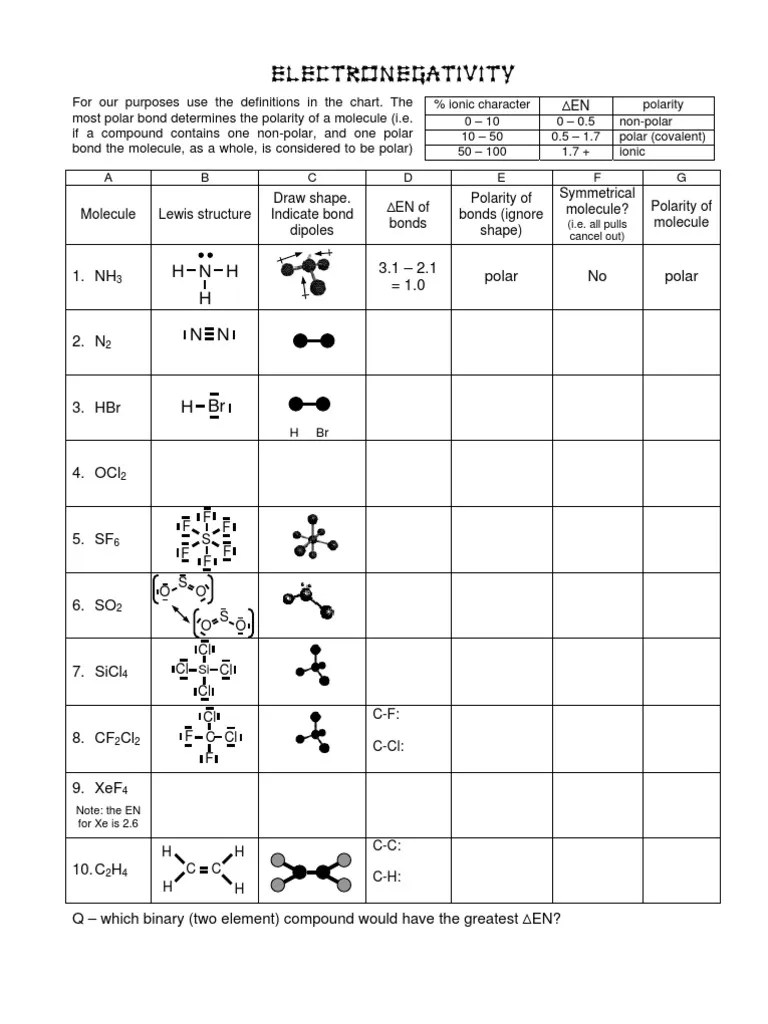
Polarity Of Molecules Worksheet
Polarity Of Molecules Worksheet - Which atom has the partial negative charge? Determine the polarity of chemical bonds between atoms using the concept of electronegativity; Ccl 2 f 2 d. Worksheets are lewis structures shapes and polarity, molecular polarity, chemistry 20 lesson 11 ele. Web determine based on electronegativity and symmetry if the following molecules are polar or nonpolar. Web the two factors that determine the polarity of a molecule are the presence of polar bonds within the molecule and the geometry (shape) of the molecule. Electronegativity difference in between 0.4 and 2.0 (nonmetal + nonmental further apart on the periodic table) ionic: Web a polar molecule is a molecule that has an overall polarity due to the shape of the molecule and/or the presence of bond dipoles within the molecule. Web showing 8 worksheets for polarity of molecules. Web displaying 8 worksheets for polarity of molecules.
Use the presence or absence of a dipole moment as an aid to deducing the structure of a given compound. Change the bond angle to see how shape affects polarity. Polar bonds are caused by unequal sharing of electrons in a bond due to a difference in electronegativity of 0.5 units or greater between the atoms in the bond. Web when is a molecule polar? Familiarize with the different molecular shapes; The units of dipole moment are the debye (d), defined as 3.34. Web a polar molecule is a molecule that has an overall polarity due to the shape of the molecule and/or the presence of bond dipoles within the molecule.
Web for the following molecules, using valence bond theory, show the orbital overlap of the following molecules. Polar bonds are caused by unequal sharing of electrons in a bond due to a difference in electronegativity of 0.5 units or greater between the atoms in the bond. General chemistry start typing, then use the up and down arrows to select an option from the list. For each of the following pairs of compounds, determine which is most polar based on their lewis structures. Web explain the concepts of polar covalent bonds and molecular polarity.
Polarity Worksheet Worksheet Polarity Of Bonds Answers 221 Best
Worksheets are lewis structures shapes and polarity, molecular polarity, chemistry 20 lesson 11 electr. Methyl chloride (chcl3) or methyl bromide (chbr3) since chlorine is more electronegative than bromine, the molecule has a higher polarity. How to determine polarity in a molecule. Predict whether a molecule will possess a dipole moment from the molecular formula or structure. Which atom has the.
Polarity Worksheet
The lewis diagrams for the two compounds are shown below. The units of dipole moment are the debye (d), defined as 3.34. Polarity of molecules (1717203) polarity of molecules. Indicate the hybridization of of all orbitals, and the type of overlap used. Web determine based on electronegativity and symmetry if the following molecules are polar or nonpolar.
Polarity Of Molecules Worksheet Answers Agaliprogram
Which of the following best describes the term dipole. See how the molecule behaves in an electric field. Worksheets are lewis structures shapes and polarity, molecular polarity, chemistry 20 lesson 11 electr. Differentiate polar and nonpolar bonds; For each of the following pairs of compounds, determine which is most polar based on their lewis structures.
Ionic Bonding Lewis Dot Structure Worksheet
See how the molecule behaves in an electric field. Web lewis structures, vsepr, polarity, im forces. Determining bond & molecular polarity: Ccl 2 f 2 d. Water or hydrogen sulfide (h2s)
Electronegativity Worksheet Free Download
Web a polar molecule is a molecule that has an overall polarity due to the shape of the molecule and/or the presence of bond dipoles within the molecule. Web lewis structures, vsepr, polarity, im forces. Use the presence or absence of a dipole moment as an aid to deducing the structure of a given compound. The units of dipole moment.
molecular geometry practice worksheet
Worksheets are lewis structures shapes and polarity, molecular polarity, chemistry 20 lesson 11 electr. Indicate the hybridization of of all orbitals, and the type of overlap used. See how the molecule behaves in an electric field. Methyl chloride (chcl3) or methyl bromide (chbr3) since chlorine is more electronegative than bromine, the molecule has a higher polarity. Water or hydrogen sulfide.
Lewis Structure Practice Worksheet
Science > ap®︎/college chemistry > molecular and ionic compound structure and properties > vsepr. Polar bonds are caused by unequal sharing of electrons in a bond due to a difference in electronegativity of 0.5 units or greater between the atoms in the bond. Indicate the hybridization of of all orbitals, and the type of overlap used. Web determine based on.
Electronegativity Worksheet Answers
See how the molecule behaves in an electric field. Worksheets are lewis structures shapes and polarity, molecular polarity, chemistry 20 lesson 11 ele. Web lewis structures, vsepr, polarity, im forces. Use the presence or absence of a dipole moment as an aid to deducing the structure of a given compound. Hint as big lines of atoms.
Polarity of Molecules YouTube
Physical science (1061445) main content: Identify the major intermolecular force in each compound. Science > ap®︎/college chemistry > molecular and ionic compound structure and properties > vsepr. Predict whether a molecule will possess a dipole moment from the molecular formula or structure. Cf 2 h 2 e.
Polarity Of Molecules Worksheet - Two different compounds have the chemical formula xef a 2 cl a 2. Familiarize with the different molecular shapes; Use the presence or absence of a dipole moment as an aid to deducing the structure of a given compound. Web for the following molecules, using valence bond theory, show the orbital overlap of the following molecules. Which of the following best describes the term dipole. Which atom is more electronegative? What type of intermolecular force exists between polar molecules? Web showing 8 worksheets for polarity of molecules. Determine the polarity of chemical bonds between atoms using the concept of electronegativity; Hint as big lines of atoms.
Familiarize with the different molecular shapes; Which atom has the partial negative charge? The units of dipole moment are the debye (d), defined as 3.34. Web when is a molecule polar? Web explain how dipole moments depend on both molecular shape and bond polarity.
Physical science (1061445) main content: Hint as big lines of atoms. Determine the polarity of chemical bonds between atoms using the concept of electronegativity; Web a polar molecule is a molecule that has an overall polarity due to the shape of the molecule and/or the presence of bond dipoles within the molecule.
Web Displaying 8 Worksheets For Polarity Of Molecules.
Change the bond angle to see how shape affects polarity. Water or hydrogen sulfide (h2s) Electronegativity difference is above 2.0 (metal + nonmetal) Identify the major intermolecular force in each compound.
Change The Electronegativity Of Atoms In A Molecule To See How It Affects Polarity.
Web a polar molecule is a molecule that has an overall polarity due to the shape of the molecule and/or the presence of bond dipoles within the molecule. Web polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. Differentiate polar and nonpolar bonds; Ccl 2 f 2 d.
Web Explain The Concepts Of Polar Covalent Bonds And Molecular Polarity.
What type of intermolecular force exists between polar molecules? For each of the following pairs of compounds, determine which is most polar based on their lewis structures. How to determine polarity in a molecule. Polarity of molecules (1717203) polarity of molecules.
Methyl Chloride (Chcl3) Or Methyl Bromide (Chbr3) Since Chlorine Is More Electronegative Than Bromine, The Molecule Has A Higher Polarity.
Web the two factors that determine the polarity of a molecule are the presence of polar bonds within the molecule and the geometry (shape) of the molecule. Web determining polarity through molecular structure and bond polarities | pdf | chemical polarity | covalent bond. Indicate the hybridization of of all orbitals, and the type of overlap used. Web when is a molecule polar?