Isotopes Worksheet With Answers
Isotopes Worksheet With Answers - 12c 14 13c c a. Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides. Web isotopes are versions of the same element. The numbers 12, 13, and 14 refer to the mass number d. 32 protons, 38 neutrons, 32 electrons. Which isotope of lead is likely to be the most abundant? Web isotopes of an element are atoms with the same atomic number but different mass numbers; Isotopes are atoms of the same element with different atomic masses. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. Atoms of a given element which have the same number of protons but different numbers of neutrons are called.
To find the most naturally occurring mass, you should round the atomic mass from the periodic table to the nearest whole number. Fill in the table with the correct information. Atoms with same atomic number (same protons),but different # of neutrons. What do all isotopes of an element have in common? Isotopes are versions of the same element. How can you tell isotopes of the same element apart? Identify which among the isotope symbols below is incorrect.
The numbers 12, 13, and 14 refer to the _____z d. Here are three isotopes of an element: The number indicates the isotope’s mass number. The numbers 12, 13, and 14 refer to the mass number d. Which part(s) of the isotope symbol was the most helpful in answering part a of this question?
Isotopes Worksheet Answers
Here are three isotopes of an element: Isotopes are atoms of the same element with different atomic masses. How many protons and neutrons are in the first isotope? Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. The numbers 12, 13, and 14 refer to the mass number d.
50 Atoms And Isotopes Worksheet Answers
The number 6 refers to the atomic number c. Ib chemistry on mole concept ram rmm isotopes and empirical. Web most of the worksheets below focus on knowledge of protons, neutrons, and electrons, though some do include isotopes and ions. How can you tell isotopes of the same element apart? Which part(s) of the isotope symbol was the most helpful.
Isotopes Ions And Atoms Worksheet 2 Answer Key 2019 Rocco Worksheet
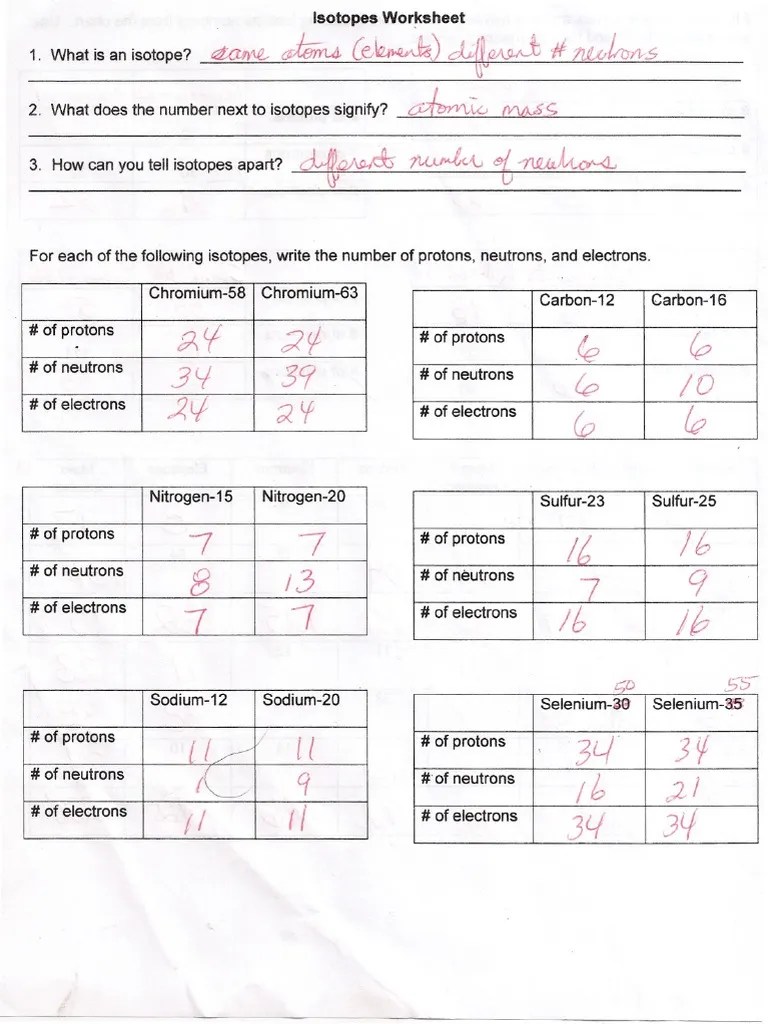
What does the number next to isotopes signify? Which part(s) of the isotope symbol was the most helpful in answering part a of this question? These isotopes are neutral (charge = 0). What is the isotope notation of the element that has an atomic number of 24 and a mass number of 52? _____ _____ for each of the following.
Isotopes Worksheet Answers Printable Word Searches
32 protons, 38 neutrons, 32 electrons. Isotopes of an element, therefore, differ from each other only in the number of neutrons within the nucleus. Identify which among the isotope symbols below is incorrect. Describe the general arrangement of subatomic particles in the atom. How many protons and neutrons are in the first isotope?
Isotopes And Ions Worksheets Answers
Web isotope practice worksheet 1. How can you tell isotopes of the same element apart? Understand the relationship between the masses of isotopes and the atomic weight of an element; 5 protons, 6 neutrons, 5 electrons. Atoms of a given element which have the same number of protons but different numbers of neutrons are called.
Chemistry Isotopes Worksheet Answers Promotiontablecovers
The number indicates the isotope’s mass number. Isotopes are versions of the same element. Web understand the structure of atoms, isotopes, and ions; Web isotopes are versions of the same element. Web isotopes are atoms of the same element with different mass numbers.
Isotopes Worksheet Answers
Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. The average atomic mass of a lead atom is 207.2 amu. The number 6 refers to the atomic number c. Answer the questions based on the above reading. Web isotopes are defined as atoms of the same element but differ in.
Average Atomic Mass Lab Gizmo Answer Key Solved Explorelearning Date
The main feature of a worksheet should be to make sure the students have the important facts written down in front of them, allowing you to build an engaging lesson around it. Ib chemistry on mole concept ram rmm isotopes and empirical. Here are three isotopes of an element: What does the number next to isotopes signify? Web use the.
Isotopes And Atomic Mass Worksheet Answers
(i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe. What does the number next to isotopes signify? 12c 14 13c c a. Ions are defined as chemical species of an atom with an electrical charge.io with a positive charge are called cations, and ions with a negative charge are called anions. Web for each.
Isotopes Worksheet With Answers - They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. Isotopes are versions of the same element. Therefore ,they are the same element, but with different masses. Web use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. What does the number next to isotopes signify? Here are three isotopes of an element: What does the number next to isotopes signify? Web isotopes of an element are atoms with the same atomic number but different mass numbers; 612 c 613 c 614 c a. Web isotopes worksheets and full answers | teaching resources.
Web isotopes of an element are atoms with the same atomic number but different mass numbers; The numbers 12, 13, and 14 refer to the _____z d. What would happen if the number of protons were to change in an atom? Isotopes of an element, therefore, differ from each other only in the number of neutrons within the nucleus. Understand the relationship between the masses of isotopes and the atomic weight of an element;
Web use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. The numbers 12, 13, and 14 refer to the mass number d. Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. Web for each isotope shown, give the number of protons, neutrons, and electrons.
You Would Get A Different Element.
Fill in the table with the correct information. The number 6 refers to the _____ c. Calculate relative atomic mass using isotopic abundances. How can you tell isotopes of the same element apart?
The Average Atomic Mass Of A Lead Atom Is 207.2 Amu.
Consult the following list of isotopes symbols: How many protons and neutrons are in the first isotope? Identify which among the isotope symbols below is incorrect. All the pb’s and br’s.
Web Use The Sim To Learn About Isotopes And How Abundance Relates To The Average Atomic Mass Of An Element.
Web isotopes are atoms of the same element with different mass numbers. How many protons and neutrons are in the second isotope? Isotopes are versions of the same element. Understand the relationship between the masses of isotopes and the atomic weight of an element;
5 Protons, 6 Neutrons, 5 Electrons.
What contribution did these scientists make to atomic models of the atom? Web how can you tell isotopes apart? They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. The number indicates the isotope’s mass number.