How Can You Tell Isotopes Of The Same Element Apart
How can you tell isotopes of the same element apart - Web answer (1 of 3): Web what is an isotope? Web and a smaller few hydrogen nuclei are substituted by a nuclide with 2 neutrons, i.e. Web in contrast, the number of neutrons for a given element can vary. Web isotopes differ in the total number of neutrons present in their respective atomic nuclei. Web how can you tell isotopes of the same element apart? Web how can you tell isotopes of the same element apart? Web 3 people found it helpful. How can you tell isotopes apart? Web if you are breaking down a molecule into its smallest individual pieces, you get elements of the same type.
Atoms of the same element with the same number of protons, but different number of neutrons. How can you tell isotopes of the same element apart?. Web most atoms have isotopes that occur naturally. They will have a different mass number and different number of. Web atoms of the same element must have the same number of protons, but they can have different numbers of neutrons.
Isotopes of the Same Element Have Different Numbers of Neutrons The
Forms of the same atom that differ only in their number of neutrons are called isotopes. Web what is an isotope? Web if you are breaking down a molecule into its smallest individual pieces, you get elements of the same type.
OXYGENE 16 18 TELECHARGER Morciougagarstec
Web the three are all isotopes of hydrogen. Web all isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemistry. Because they contain different numbers of neutrons,.
Hunting for rare isotopes The mysterious radioactive atomic nuclei
Web how can you tell isotopes of the same element apart? Web answer (1 of 3): Web in contrast, the number of neutrons for a given element can vary.
How Do You Calculate The Number Of Neutrons In An Element The atomic
Number of neutrons is different. They will have a different mass number and different number of. Web and a smaller few hydrogen nuclei are substituted by a nuclide with 2 neutrons, i.e.
PPT Atoms with the same atomic number but with different atomic
Number of neutrons is different.isotopes are chemical elements with same atomic number, but different. Web how can you tell isotopes of the same element apart? Web science chemistry isotope how can you tell isotopes apart?
Mass Number — Definition & Overview Expii
Web the isotopes of a given element always contain the same number of protons and therefore occupy the same place on the. Number of neutrons is different. Web most atoms have isotopes that occur naturally.
Isotopes of the Same Element Have Different Numbers of Neutrons The
Number of neutrons is different.isotopes are chemical elements with same atomic number, but different. Because they contain different numbers of neutrons,. Web all isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemistry.
Web the three are all isotopes of hydrogen. Web atoms of the same element must have the same number of protons, but they can have different numbers of neutrons. Web if you are breaking down a molecule into its smallest individual pieces, you get elements of the same type. Web 3 people found it helpful. How can you tell isotopes of the same element apart?. How can you tell isotopes apart? Web science chemistry isotope how can you tell isotopes apart? Web and a smaller few hydrogen nuclei are substituted by a nuclide with 2 neutrons, i.e. They will have a different mass number and different number of. Web chemically, all three are indistinguishable, because the number of electrons in each of these three isotopes.
Web the isotopes of a given element always contain the same number of protons and therefore occupy the same place on the. Web how can you tell isotopes of the same element apart? Web how can you tell isotopes of the same element apart? Web in contrast, the number of neutrons for a given element can vary. An isotope is an atom with a different number of neutrons, but the same. Web isotopes differ in the total number of neutrons present in their respective atomic nuclei. Web most atoms have isotopes that occur naturally. An isotope is a particular element (or atom species) with particular number of neutrons. Thus they have different mass. Web answer (1 of 3):
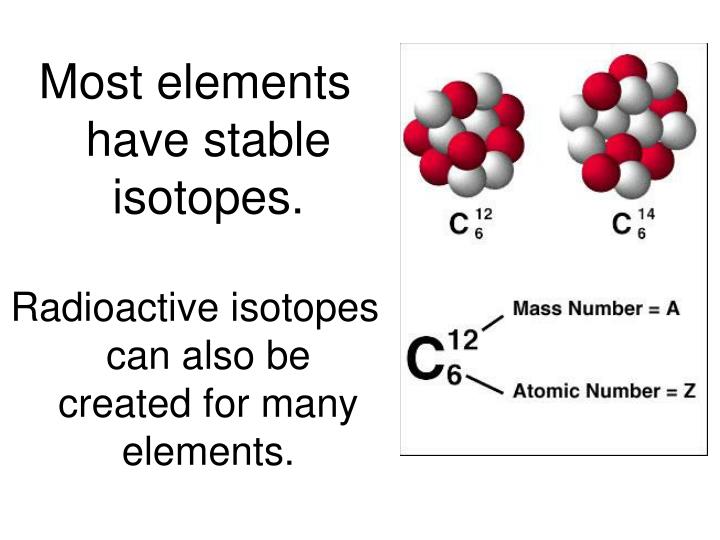
Number of neutrons is different.isotopes are chemical elements with same atomic number, but different. Forms of the same atom that differ only in their number of neutrons are called isotopes. Atoms of the same element with the same number of protons, but different number of neutrons. Web isotopes are atoms of the same element with different numbers of neutrons. Web the term isotopes (originally also isotopic elements, now sometimes isotopic nuclides) is intended to imply comparison (like. Because they contain different numbers of neutrons,. As you can see, they have the same atomic number, or number of protons, (number at. Web what is an isotope? Number of neutrons is different. The isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons.